- API
- Ivabradine hydrochlorid
- Rivaroxaban
- Apixaban
- Dabigatran etexilate
- Safinamide
- Ticlopidine hydrochloride
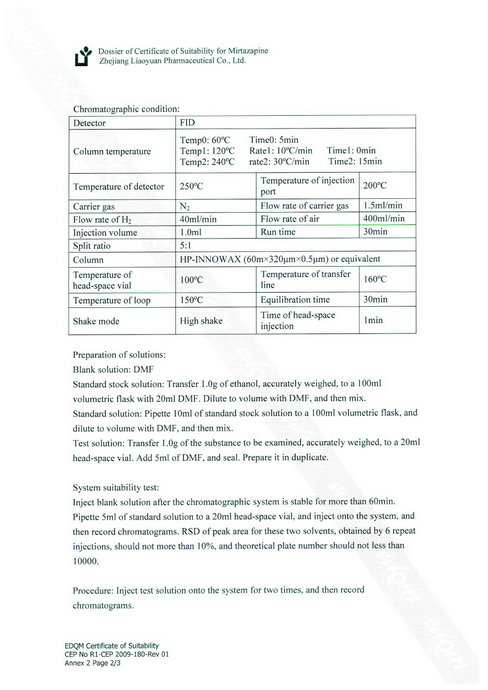
- Mirtazapine
- Clopidogrel bisulfate
- Duloxetine hydrochloride
- Rebamipide
- Intermediates
- Duloxetine intermediate
- Clopidogrel intermediate
- Mirtazapine intermediates
- Dabigatran etexilate intermediates
- Lurasidone intermediates
- Bepotastine intermediates
- Asenapine Intermediates
- Drotaverine intermediates
- Prasugrel Intermediates
- Ivabradine、Verapamil, Denopamine intermediates
- Moxifloxacin intermediate
- Ticagrelor intermediate <Under Development>
- Canagliflozin intermediate
- Ivabradine intermediate
Address: Zhejiang Provincial Chemical and Medical Raw Material Base Linhai Zone, Duqiao Town, Linhai City, Zhejiang Province, 317016, China
Tel / Fax: 0576-85588211
Tel / Fax: 0576-85588211
 [2016-12-22] Certificate of suitability No. R1-CEP 2004-109-Rev...
[2016-12-22] Certificate of suitability No. R1-CEP 2004-109-Rev... [2016-12-22] Certificate of suitability No. R1-CEP 2009-180-Rev...
[2016-12-22] Certificate of suitability No. R1-CEP 2009-180-Rev... [2016-12-22] JP Ticlopidine_HCl_DMF_registration_No
[2016-12-22] JP Ticlopidine_HCl_DMF_registration_No [2016-12-22] J-DMF Rebamipide(2012.03.30)(Fujikawa)
[2016-12-22] J-DMF Rebamipide(2012.03.30)(Fujikawa) [2016-12-22] Certificate of suitability No. R0-CEP 2015-156-Rev...
[2016-12-22] Certificate of suitability No. R0-CEP 2015-156-Rev... [2016-12-22] GR I N MF登録証クロピドグレル(N)
[2016-12-22] GR I N MF登録証クロピドグレル(N) [2016-12-22] Certificate of suitability No. R0-CEP 2014-239-Rev...
[2016-12-22] Certificate of suitability No. R0-CEP 2014-239-Rev... [2012-02-25] This COS certificate had been upadated in January ...
[2012-02-25] This COS certificate had been upadated in January ... [2012-02-25] A attestation of inspection was issued by EDQM & H...
[2012-02-25] A attestation of inspection was issued by EDQM & H... [2012-02-25] The company was granted the GMP Certificate by Da...
[2012-02-25] The company was granted the GMP Certificate by Da... [2012-02-25] The company updated the ISO 14001 Certificate on 2...
[2012-02-25] The company updated the ISO 14001 Certificate on 2... [2012-02-25] The company updated the ISO 9001 Certificate on 2n...
[2012-02-25] The company updated the ISO 9001 Certificate on 2n... [2012-02-25] DMF registration certificate of Ticlopidine HCl (J...
[2012-02-25] DMF registration certificate of Ticlopidine HCl (J... [2012-02-25] March 22, 2007,granted “Accreditation of Foreign M...
[2012-02-25] March 22, 2007,granted “Accreditation of Foreign M... [2012-02-25] Imiquimod DMF DMF No.: 20256, submitted on 28 Janu...
[2012-02-25] Imiquimod DMF DMF No.: 20256, submitted on 28 Janu... [2012-02-25] 1-(3-hydroxymethylpyridyl-2)-2-phenyl-4-methylpipe...
[2012-02-25] 1-(3-hydroxymethylpyridyl-2)-2-phenyl-4-methylpipe... [2012-02-25] A COS issued by EDQM on July 7, 2006. Certificate ...
[2012-02-25] A COS issued by EDQM on July 7, 2006. Certificate ... [2012-02-25] Inspected by local authority, Zhejiang Province Fo...
[2012-02-25] Inspected by local authority, Zhejiang Province Fo...